| Phenols are found in some of the healthiest foods and supplements we consume. The foods highest in phenols are all berries, dark-skinned grapes, pomegranates and other fruits with dark red and purple pigments. In many cases it's the phenols that make them healthy. There's a flip side to phenols though. It's a perfect example of "too much of a good thing". I'll explain here all of the health benefits of phenols, how the body processes them, how things can go wrong, symptoms of too many phenols and what to do about it. |
What are phenols?
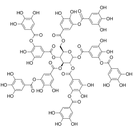
| Phenols are technically chemical compounds that contain a hydroxyl group (-OH) attached to an aromatic hydrocarbon group (ring of carbons). They are found as simple phenols (image 1) and polyphenols that contain many carbon rings (image 2). Because they can take many shapes and sizes they have many different functions within our bodies and the environment. The phenols discussed here are those found in food that have health benefits. There are also synthetic phenols, phenols made in the body (dopamine, epinephrine, estrogen, et al) and phenols from the environment that have estrogen and endocrine disrupting properties. |
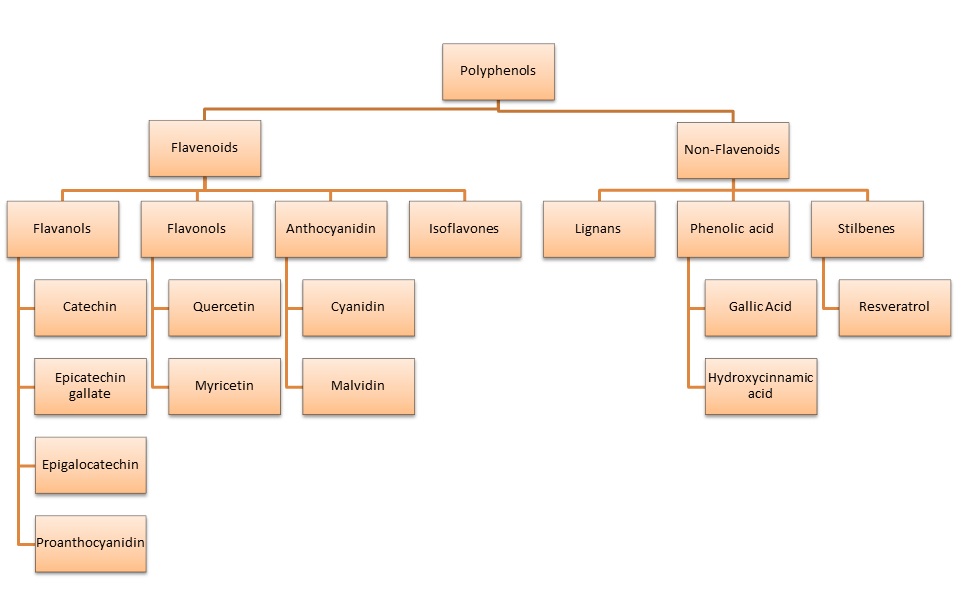
Healthy polyphenols from food can be categorized into flavenoids and non-flavenoids (image 3). Polyphenols are abundant micronutrients found in our diet. They've been linked to preventing degenerative diseases like cardiovascular disease, cancer and dementia (1,2,3). Their exact mechanism of action isn't completely understood. What is known is that they have anti-inflammatory and anti-oxidant properties (4,5,6).
In addition to the above mentioned benefits polyphenols have been studied for their effect on those with Down syndrome. These studies give some insight into the possibility that polyphenols have epigenetic properties and impact the expression of the DYRK1A gene, found on chromosome 21. This gene codes for the enzyme Dual Specificity Tyrosine Phosphorylation Regulated Kinase 1A. This enzyme plays a roll in cell proliferation (increase in number of cells) and brain development. As with many genes, over-expression and under-expression of the same gene can be a problem. Given that those with Down syndrome have an extra copy of chromosome 21 the over-expression of DYRK1A is considered to be a strong candidate gene for learning defects associated with Down syndrome (7).
The polyphenol that has been most tested for it's impact on those with Down syndrome due to it's known ability to inhibit DYRK1A expression is EGCG (Epigallocatechin gallate). Researchers in Spain tested EGCG in children with Down syndrome and found it "significantly reverses cognitive deficits in a pilot study in DS individuals with effects on memory recognition, working memory and quality of life" (8). EGCG has also been shown to play a role in preventing Alzheimer's disease which occurs in 100% of those with Down syndrome (9).
The polyphenol that has been most tested for it's impact on those with Down syndrome due to it's known ability to inhibit DYRK1A expression is EGCG (Epigallocatechin gallate). Researchers in Spain tested EGCG in children with Down syndrome and found it "significantly reverses cognitive deficits in a pilot study in DS individuals with effects on memory recognition, working memory and quality of life" (8). EGCG has also been shown to play a role in preventing Alzheimer's disease which occurs in 100% of those with Down syndrome (9).
More evidence exists supporting the impact that polyphenols have on genetic expression. In 2013 Pan reported, "Over the past few decades, polyphenols, which are widely present in foods such as fruits and vegetables, have been shown to exhibit a broad spectrum of biological activities for human health. Polyphenols reverse adverse epigenetic regulation by altering DNA methylation and histone modification, and they modulate microRNA expression or directly interact with enzymes that result in the reactivation of silenced tumor suppressor genes or the inactivation of oncogenes."(10). Susanne Henning and her team at the UCLA School of Medicine also reported in 2013 that EGCG does, in fact, inhibit DNA methylation (11). Cancer ican be prevented by inhibiting DNA methylation because methylation can block the expression os tumor suppressor genes. You can read more about DNA Methylation in by blog post here.
Another popular polyphenol that has a lot of research supporting it's use in neurodegenerative diseases is resveratrol (12). Resveratrol is found in red grapes and Japanese knotweed. It, too, has been tested in those with Down syndrome, but studies are few. Researchers in Italy have reported "the natural polyphenol resveratrol, which displays a neuroprotective action in various human diseases but never tested in DS, restores oxidative phosphorylation efficiency and mitochondrial biogenesis."(13). Many parents have started using resveratrol in their children with Down syndrome and are reporting immediate and clear increases in speech and cognition when starting resveratrol. Unfortunately, no published studies exist currently to support this.
When Do Phenols Cause Harm?
Ultimately, the multiple ways that dietary polyphenols positively impact human health cannot be denied. But, is there a point that they can cause any harm or do they have any side effects? The answer is, "Yes".
Phenols need to be processed, metabolized and modified within the body in order to work properly. The main enzyme that's involved in the processing of phenols is phenol sulfotransferase (PST). This enzyme is heavily dependent on sulfur as you might be able to tell from it's name. So, the intersection of sulfation and phenol metabolism within the body is an important one. I'll start by talking about sulfation.
Sulfation can activate or inactivate a wide range of biological compounds and any change in the supply of sulfate can have potentially serious consequences within the body. Sulfation is the transfer of a sulfate group from 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to a compound by a family of sulfotransferase enzymes, like PST. Once that sulfate group is transferred the compund is activated. The process of obtaining sulfur from our diet involves several steps that each require a different enzyme and don't necessarily occur in this order.
- Sulfate is absorbed in the gastrointestinal tract via a sodium-sulfate symporter, meaning sodium is needed for sulfate to be absorbed. These transporters can become saturated or filled up during each meal, so eating sulfur containing foods with each meal is the ideal way to raise sulfate levels within the blood. In addition, some bacteria in the gut can convert sulfate to sulfide, making it unavailable. So, sulfate can be difficult to absorb in the gut.
- Two sulfur containing amino acids obtained from the diet are methionine and cysteine. Obtaining sulfur from cysteine first involves cysteine being converted to cysteine sulfinic acid by the cysteine dioxygenase (CDO) enzyme. This enzyme is B6, iron and histidine dependent.
- Cysteine sulfinic acid can then become either taurine or sulfite. The sulfoxidation enzyme (SUOX) converts sulfite, which is toxic, to sulfate. This enzyme is very dependent on the mineral molybdenum.
You can see from these steps that if sulfur in the diet, sulfur absorption, B6 or molybdenum are deficient this can create problems in a wide arrary of biological activities, including the activity of the PST enzyme. When this enzyme is not supported by adequate levels of sulfate it creates an overload of phenols that are not processed properly. Unfortunately there is no test for this. It's based solely on symptoms, however there are some labs that can give clues to an impaired sulfation system.
| Symptoms of phenol overload include:
|
Some clues to sulfation issues with in the body on labs can be a high taurine in the urine. This is termed "taurine wasting". This is the body's means of removing toxic sulfite when it isn't converted properly to sulfate. The body "chooses" taurine because it is high in sulfur. This creates an actual taurne deficiency. Supporting the SUOX enzyme with molybdenum can help to rememdy this. In fact, researchers in Germany found a 20-fold increase in urinary taurine levels in those who were deficient in molybdenum (14). If B6 deficiency is seen on an organic acid test in an elevated xanthuernic acid, kynurenic/quinolinic acid then supplementing with B6 can also help support the CDO enzyme.
In addition to phenol overload, parents using polyphenols for their children should also know that phenols are strong infibitors of iron absorption (15) which can already be a problem for some children, especially those with gastrointestinal disorders. I often recommend to not start EGCG or resveratrol until ferritin is closer to 40 ng/mL or at least to take an iron supplement when needed, but as far from taking the phenolic compound as possible (i.e., iron at bedtime and EGCG with breakfast). As well, EGCG inhibits the catechol-o-methyltransferase enzyme (COMT) that breaks down estrogen, epinephrine and norepinephrine (adrenalin) (16). Parents should be mindful of a potential increase in adrenalin when using EGCG.
Lastly, there are some that may have a mutation in their PST enzyme (17), making it work more slowly despite sufficient sulfate. It's been estimated in unpublished data by Dr. Rosemary Waring that approximately 80-90% of children with autism struggle with the function of this enzyme either because of low sulfate levels or because of a defect in this enzyme that detoxifies environmental toxins, food dyes, additives, some medications, phenols made by the body and phenols obtained in the diet.
How to Support Phenol Metabolism
The first step is to remove or greatly reduce all high phenol foods and supplements for at least 2 weeks to see if symtpoms improve. If they do, avoiding high phenol foods and supplements does not have to last forever. The goal is to support the PST enzyme by increasing sulfate blood levels. Because sulfate can be difficult to absorb in the GI tract using epsom salt baths with magnesium sulfate on a nightly basis is an excellent way to increase sulfate in the body. As well, taking molybdenum, B6 and magnesium can also support the sulfation pathways.
Click on the image below to open a printable pdf to refer to often during the 2 weeks of avoiding foods high in phenols:
In addition to the above steps there also is an enzyme that can be taken as a supplement that helps process phenols. It's xylanase. The products below have been used successfully by many parents when helping their children with phenol overload. I don't recommend simply starting this enzyme. Removing high phenol foods and supplements for two weeks is the first step to lower the phenol load within the body. Then slowly introduce high phenol foods with one of these enzymes. Only after no reactions are seen should high phenol supplements like EGCG and resveratrol be reintroduced.
Ultimately, parents should know that just because a supplement or compound comes from nature does not mean that it doesn't have side effects, especially when given in higher doses than would be obtained from food as in the case of supplements. The benefits from these compounds can be great when the child's body is ready for it and all necessary cofactors are in place for it to work optimally. We all want to ensure that our children are given supplements that are helping them and not causing harmful side effects.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC2835915/Kampa M., Nifli A.P., Notas G., Castanas E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007;159:79–113.
- Habauzit V, Morand C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: an update for clinicians. Therapeutic Advances in Chronic Disease. 2012;3(2):87-106.
- Hügel HM, Jackson N. Polyphenols for the prevention and treatment of dementia diseases. Neural Regeneration Research. 2015;10(11):1756-1758.
- Santangelo C., Varì R., Scazzocchio B., Di Benedetto R., Filesi C., Masella R. Polyphenols, intracellular signalling and inflammation. Annali-istituto Super. di Sanita. 2007;43:394
- Joseph S, Edirisinghe I, Burton-Freeman B. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr. 2016;56(3):419-44.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2009;2(5):270-278.
- Soppa U, Schumacher J, Florencio Ortiz V, Pasqualon T, Tejedor FJ, Becker W. The Down syndrome-related protein kinase DYRK1A phosphorylates p27Kip1 and Cyclin D1 and induces cell cycle exit and neuronal differentiation.Cell Cycle. 2014;13(13):2084-2100.
- De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farré M, Dierssen M. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Molecular Nutrition & Food Research. 2014;58(2):278–288.
- Xicota L., Rodríguez-Morató J., Dierssen M., de la Torre R. (2015). Potential role of (-)-epigallocatechin-3-gallate (EGCG) in the secondary prevention of Alzheimer disease. Curr. Drug Targets. Curr Drug Targets. 2015 Aug 25. [Epub ahead of print]
- Pan MH, Lai CS, Wu JC, Ho CT. Epigenetic and disease targets by polyphenols. Curr Pharm Des. 2013;19(34):6156-85.
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Molecular neurobiology. 2010;41(2-3):375-383.
- Henning SM, Wang P, Carpenter CL, Heber D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics. 2013;5(6):729-741.
- Valenti D, de Bari L, de Rasmo D, Signorile A, Henrion-Caude A, Contestabile A, Vacca RA.The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model.Biochim Biophys Acta. 2016 Jun;1862(6):1093-104.
- Belaidi AA1, Schwarz G. Molybdenum cofactor deficiency: metabolic link between taurine and S-sulfocysteine.Adv Exp Med Biol. 2013;776:13-9.
- Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? International Journal of Molecular Sciences. 2011;12(9):5592-5603.
- Chen D., Wang CY., Lambert JD., Ai N., Welsh WJ., Yang CS.. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol 2005; 69(10):1523-31
- Price RA, Spielman RS, Lucena AL, Van-Loon JA, Maidak BL, Weinshilboum RM. Genetic Polymorphism for Human Platelet Thermostable Phenol Sulfotransferase (Ts Pst) Activity. Genetics. 1989;122(4):905-914.







 RSS Feed
RSS Feed